Fluorescence
An exploration of fluorescence as a material for creative & interaction design
Photo featured by ATLAS — The director of ATLAS, Mark Gross, interacting with the final demonstration of my material exploration
purpose
To explore the creative affordances of fluorescence and its potential for creative and/or interaction design. As a material that can often be used in kitschy or obvious ways — such as rave culture, Hollywood depictions of crime analysis, and children’s crafts — I hoped to use this material in designs that are unconventional and speak to the potential of fluorescence in meaningful design. I was not wholly successful in avoiding the inherent kitsch of fluorescence (after all, we are talking about a material that glows in black lights), however I do believe my final prototypes illustrate the potential for the use of fluorescence in education, in visual arts and design, and in the study of fluid dynamics and flow visualization.
background
Through research and experimentation, I hoped to systematically explore the creative affordances and limitations of fluorescence in design. Fluorescence is a form of luminescence, in which a substance that has absorbed light or other electromagnetic radiation then emits light — usually at a longer wavelength and lower energy than the absorbed radiation. Regular coloration (such as the color of a red sweater) occurs because the object or substance absorbs some wavelengths of light but not others. Fluorescent colors, however, absorb light in the ultraviolet portion of the electromagnetic spectrum — light that is invisible to the human eye — and emit light in the visible color spectrum. This gives fluorescent substances a distinct “glow,” which is most visible when exposed to ultraviolet light.
For this material exploration, I primarily worked with distilled fluorescein, an organic compound and dye that is orange or dark red in color with a bright yellowish-green fluorescence. Fluorescein is prepared by heating phthalic anhydride and resorcinol over a zinc catalyst, and it crystallizes as a deep red powder, which is often combined with water or ethanol to form a tracer dye. Fluorescein is used as an indicator and tracer in several contexts: in medicine (particularly as a diagnostic tool in ophthalmology and optometry to diagnose corneal abrasions, ulcers, and infections), in the military (particularly in water rescue as a tracer dye), and in hydrology and water treatment (particularly in tracer tests to help in understanding water flow of both surface waters and groundwater or in leak detection). Fluorescein is also used in the study of plants, as fluorescent dye can be used to monitor and study plant vasculature, in hydrostatic testing of subsea oil and gas pipelines, and as a color additive in cosmetics.
A more common, everyday interaction with fluorescein is when one uses a yellow highlighter to mark a sentence on the page of a book. The highlighter ink, which is composed of about 5% fluorescein, absorbs light, both visible and ultraviolet, “exciting” the electrons to a higher energy state. The electrons do not remain in this higher energy state, but quickly “relax” back to their original state, releasing the excess energy in the form of light. The fluorescence of the ink is due to this longer wavelength of emitted light, visible to the human eye and giving highlighter ink its unique “glow.”
While I primarily worked with fluorescent tracer fluid, I also experimented with fluorescent pigment powders, and fluorescent ink that is invisible in normal lighting and visible in ultraviolet lighting. I experimented in various ways and with various materials to document the properties and affordances of fluorescence as it relates to creative and interaction design.
process outline
My experimentations with fluorescence and its relation to creative and interaction design required playing and experimenting with this material in many ways including:
Experimentation with using fluorescence as a fabric dye — both as a fabric paint and in dip and tie-dying methods;
Exploration with the solubility of fluorescein at various temperatures and in combination with various substances;
Experimentation with using the visible/invisible properties of fluorescence to hide or reveal materials or interactions;
Experimentation with fluorescent materials and their interactions with other fluids such as oil, isopropyl alcohol, and water in various forms and temperatures; and
Exploration of the fluid dynamics of fluorescein — both on its own and in combination with other materials.
While some of these experiments were ultimately failures, I did succeed in uncovering some interesting and atypical uses of fluorescence in design.
Experiments with fabric dye
I started out with attempting to dye fabric with various fluorescent materials. I painted on one shirt with fluorescent ink that is invisible in normal lighting conditions, and fluoresces under a UV light. I also attempted to tie-dye and dip-dye black fabric with the fluorescein tracer dye. Because the fluorescent materials are skin irritants, I had to “set” the dye for several hours in a combination of ice water, white vinegar, and salt before thoroughly rinsing the fabric and removing the harsh chemicals of the dye. These experiments, while somewhat interesting, did not provide me with enough of a direction for meaningful design. There are better-made and less toxic clothing materials that are fluorescent, and so I did not feel this was a useful track to pursue any further.
Experiments with temperature
Melting rate of fluorescein in room temperature water versus snowpack
I then turned to experimenting with freezing fluorescein with various amounts of reverse-osmosis (RO) filtered water to determine the expansion of the fluorescein as well as its melting rate when combined with varying amounts of water, frozen, and placed in room temperature water. The pure fluorescein did not expand when frozen, and expanded more in proportion with the amount of water it was combined with. When dropped into room temperature water, the melting rate also corresponded with the ratio of fluorescein to water. The more highly concentrated frozen cubes of fluorescein were the slowest to melt and dissolve, while the frozen cubes with more water dispersed fairly quickly.
Fluorescein frozen with water (the percentage indicates the ratio of fluorescein to water)
Frozen fluorescein (with various ratios of water) melting and dispersing in room-temperature water
As a fun side experiment with this phenomenon, and because it was snowing that day, I also compared the solubility rates of fluorescein in room-temperature water versus fluorescein added to a cup of snow from my back porch. The fluorescein, as expected, immediately dispersed in the water. In the cup of snow, however, the fluorescein did not disperse at all, but actually contracted the snow before finally melting and mixing together.
These two experiments demonstrate that the dissolution and uses of fluorescein as a tracer fluid in water could be slowed or delayed when the fluorescein is frozen or when fluorescein is placed in colder water. While these experiments did not ultimately lead to a final design solution, they were useful in determining the ways in which fluorescein interacts with other fluids (in this case water) and led to my interest in the fluid dynamics of fluorescein as it interacts with other fluids. Although I had previously understood this material to be highly visual, the most consistent findings in these experiments were that the most interesting aspect of fluorescence is in its visual applications.
Interactions with other substances
Once I had determined the effects of temperature and mixtures of fluorescence with water, I began to explore the interactions between fluorescence and other substances. Fluorescein is slightly soluble in water, ethanol, and diethyl ether, and very soluble in acetone, pyridine, and methanol. It is insoluble in benzene, chloroform, ether, or oils such as coconut oil, olive oil, or mineral oil. These attributes provide interesting visual results when fluorescein is dropped into various substances.
Because of its higher solubility in water and alcohol, fluorescein quickly mixes into both substances before eventually settling to the bottom. This process is much faster in isopropyl alcohol — in water, the fluorescein remains suspended in the water for a much longer period before eventually settling, demonstrating why it is often used as a tracer in water mechanics. In oils, such as coconut oil or mineral oil, the fluorescein does not mix at all, but rather remains in beads or clumps, eventually settling to the bottom and combining into itself.
Fluorescein-saturated isopropyl alcohol (left) and coconut oil (right)
These interactions were interesting, but I wanted to explore the visual effect of dropping fluorescein into various substances outside of simply using a dropper to vertically drop the fluorescein into glasses. In the end, I settled on four different fluids to display interactions with: water, isopropyl alcohol, coconut oil, and mineral oil.
Experiments with fluid dynamics
After weeks of “playing” with fluorescein and other fluorescent materials, I found two things to be the most interesting aspects of this material:
1) its interaction with other substances, and
2) the highly visual demonstration of fluid dynamics.
The challenge then became to display these two interactions in conjunction and in a visually engaging and interactive way. I wanted the user to be able to drop the fluorescein into the various substances, and I wanted the fluorescein’s interaction with the various substances to be the focus of the interaction. I got the idea to use clear acrylic as a vessel or background on which to display these two properties of fluorescein.
Test runs of different lasercut channels in clear acrylic
I experimented with lasercutting clear acrylic and testing various carved channels with different etched curves and lines. This process went through several iterations. Many of the original “channels” I cut did not provide an easy path down which the fluorescein could flow. Also, because the acrylic was smooth, while the etched lines had minuscule abrasions left by the laser etching, the fluorescein was more drawn to flowing down the smooth acrylic rather than remaining within the channels I had created. After many failed attempts, I realized that the fluorescein would only flow down gentler slopes, meaning no harsh angles or tight curves, and also that the fluorescein needed to be somewhat forced down the channels. The idea then emerged to utilize more than one sheet of acrylic. If I could somehow sandwich the channels between two other sheets of acrylic, the fluorescein would be forced to go in the desired direction, ultimately interacting with the other material at the bottom of the channel.
Another major challenge was gluing the clear acrylic. As it turns out, it’s extremely difficult to glue several sheets of clear acrylic together in such a way that the glue dries to be clear and watertight. I now understand why fish tanks are so expensive. Many failed prototypes involved experimenting with different types of acrylic glue. Eventually, I was able to use a capillary-action glue to create a watertight seal and dry somewhat clear, although there are still visible marks in the final designs. Theoretically, if I could have glued these in a vacuum, there would be no oxygen bubbles present. However, the final designs are the clearest and cleanest results I could achieve with the materials and time I had.
final prototypes
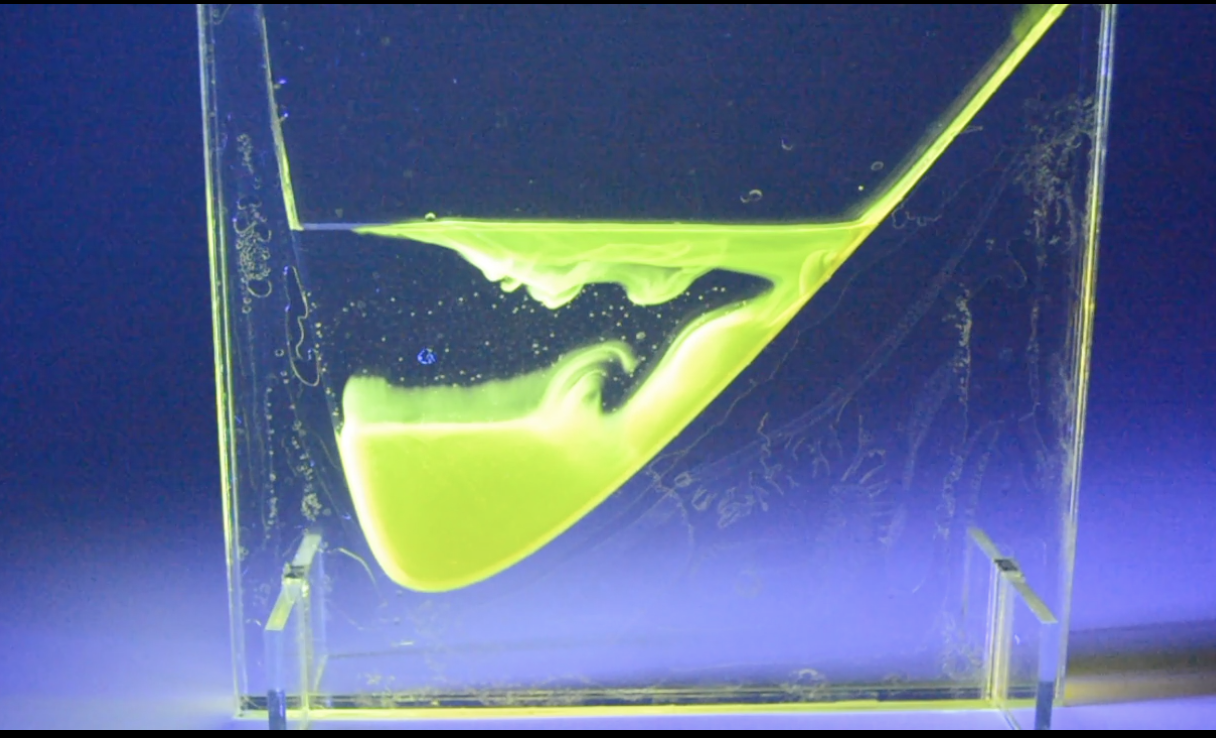
My final design consists of four acrylic prototypes, each designed to be interactive and illustrate fluorescein’s interaction with the fluid encased. Using an eye dropper, the user drops fluorescein at the top of the slope or “beaker” shape and watches as the fluorescein travels down into the fluid at the bottom. Each prototype illustrates a different flow visualization and interaction between fluorescein and another substance (further outlined below).
The designs consist of three panels of clear acrylic — two in the back and front that were 1/8” in thickness and one in the middle that was 1/4” in thickness — lasercut and sandwiched together with slots for small feet at the bottom to hold them up. They sort of resemble children’s ant farms, with the center 1/4” acrylic sheet cut to have a bowled shape in which to hold the fluid being demonstrated, and an angle leading into the bowl (with the exception of the mineral oil prototype, which is designed to mimic a beaker).
The four different prototypes were built for the interaction of fluorescein with four different fluids: water, mineral oil, isopropyl alcohol, and coconut oil.
1) Water — The water display contains a fairly steep and straight drop into the water, allowing the fluorescein to flow quickly into the water, disperse throughout, and swirl back in a suspended flow visualization. The fluorescein then remains suspended for up to thirty minutes before settling to the bottom of the acrylic window, demonstrating how useful fluorescein is in visualizing flow dynamics and as a tracer fluid.
2) Mineral Oil — This prototype was created as a sort of playful take on a science lab beaker. The user is able to drop the fluorescein into the oil, with which it doesn’t mix, and can then pick up the acrylic prototype and shake it around. The result is a sort of interactive lava lamp visualization of how diluted fluorescein does not mix with oil.
3) Isopropyl Alcohol — Because fluorescein is highly soluble in alcohol (more so than water) I wanted to create an entry slope that was gentle at first, then swiftly drops into the alcohol. This allows the viewer to see the immediate, intensive reaction between the fluorescein and the alcohol, in which the fluorescein quickly disperses then settles to the bottom.
4) Coconut Oil — The coconut oil display contains a bumpy channel, which allows for the “beads” of fluorescein to visually travel down the channel and eventually sink to the bottom, combining into one solid clump. This was an entertaining visualization of the ways in which solutions may not mix — and is slow-moving enough to demonstrate this phenomenon in a new and unique way.
conclusions and implications for design
My experiments with fluorescence have shown that this is a unique material that is capable of demonstrating fluid dynamics, flow visualization, and chemical and physical reactions in a highly visceral way. This has many implications for scientific education, design, and potential uses beyond. Think about Cloudflare’s lava lamp wall used for data encryption. The shapes and patterns that emerge from fluid dynamics are ever-changing and impossible to predict — alluding to many uses in both practical and visual design. The use of a fluorescent material such as fluorescein takes this a step further by making these flows even more apparent and visually engaging. These patterns can also be more easily traced with the use of fluorescein, allowing for further data recording and analysis.
In an educational context, I believe the use of fluorescence would be a fantastic addition to traditional scientific explanations of mixtures and solutions and the various physical and chemical interactions of materials. We can explain to children that oil and water don’t mix, but how much more engaging is this phenomenon when demonstrated with fluorescent materials? I believe that my final designs, if perfected, would be highly useful in the classroom and beyond in order to demonstrate the sort of everyday phenomena that we encounter. Children could physically interact with the materials, engaging with an abstract concept in a hands-on way.
Perhaps most obvious is the visual interest created by the flow and interactions created when interacting with my prototypes. These visuals create striking images and videos. These could be used as visuals during a musical performance, a dance performance, or simply as a relaxing video to watch while going to sleep (see the growing trend of ASMR or Autonomous Sensory Meridian Response videos on the internet). There’s something beautiful about watching a glowing green substance swirl around in water, illustrating a fluid dynamic that we may understand but not fully be able to appreciate. There’s something inherently satisfying about dropping something into coconut oil and watching it bead into little droplets that slowly work their way towards the bottom of the acrylic plate, eventually coalescing into a blob at the bottom. It’s why humans are fascinated with lava lamps, northern lights, and fireworks — bringing light to something engages the senses in a highly satisfactory and engaging way. I believe all of the possible implications of fluorescence in further interaction design are not within the scope of this project. However, I believe, and hope, that my experiments with fluorescence as a material will inspire further engagement with this material for educators and designers alike.